끊임없이 당신을 괴롭히는 알레르기 비염, 그 원인과 증상을 알고 계신가요?
다양한 알레르기비염 증상 완화에 효과적입니다.1
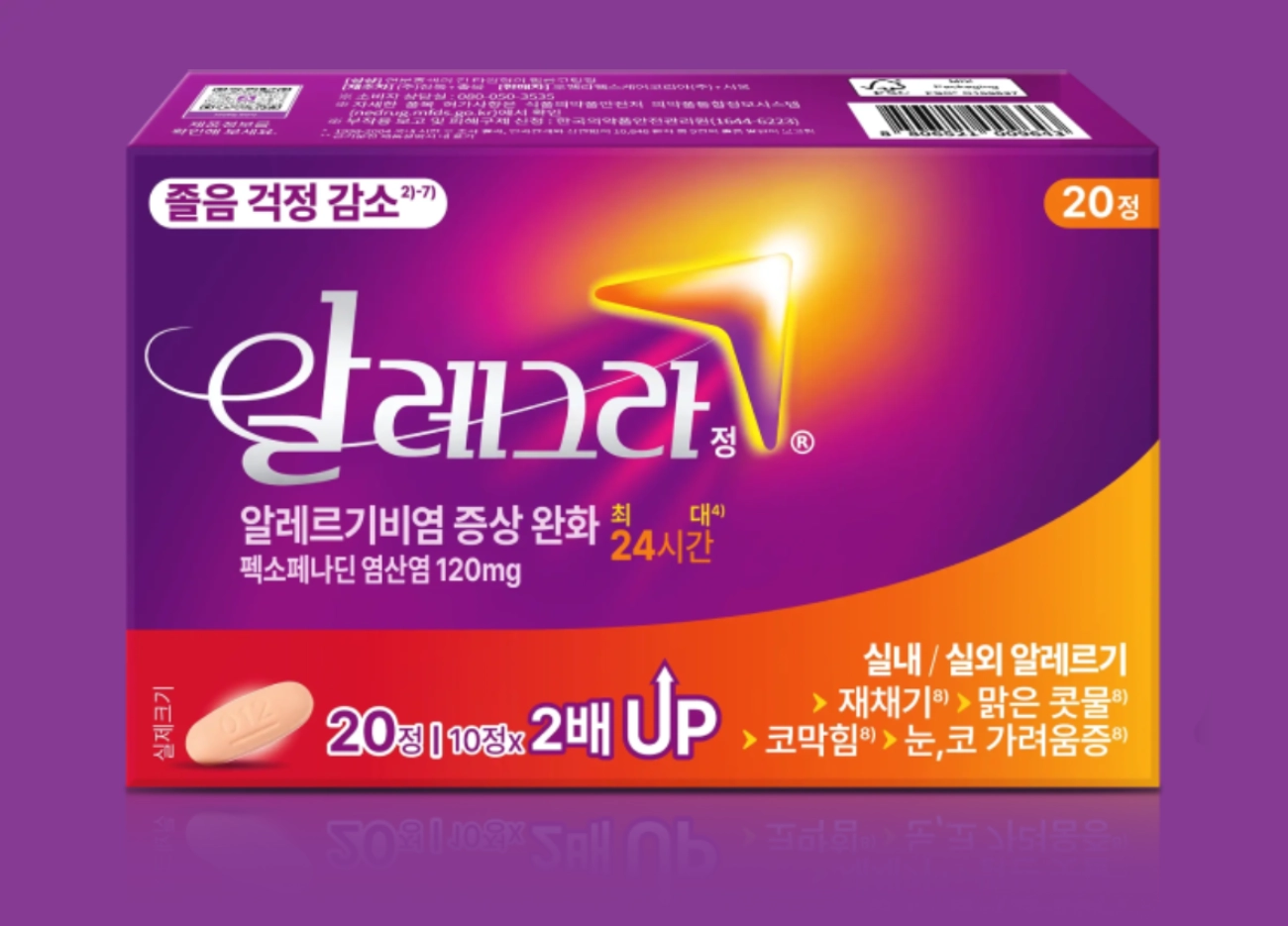
알레그라®정 120mg
알레그라®정 120 mg은 3세대 항히스타민제의 펙소페나딘(Fexofenadine) 성분을 함유한 알레르기비염 약입니다.
알레그라®정 120mg의 주성분 펙소페나딘은 알레르기비염 증상을 효과적으로 완화시켜 환자들의 삶의 질 향상에 도움을 줍니다.
알레그라®만의 장점을 알아보세요.

알레그라®는빠르게 효과가 나타났습니다.**6
알레그라®는빠르게 효과가 나타났습니다. 알레그라®는 복용 후 1시간 이내에 빠르게 효과가 나타나 답답한 코막힘, 콧물, 재채기 등을 효과적으로 완화해줍니다.

알레그라®는 졸음 유발이 적습니다. 뇌혈관 장벽을 통과하는 양이 적어 졸음, 어지러움 등의 이상반응이 적습니다.
.webp)
알레그라®는 24시간 동안 효과가 지속되었습니다.***알레그라®는 국내외 다양한 임상 연구를 통해 효과와 안전성 프로파일을 확인하였습니다.알레르기 비염은 삶의 질을 저하시키는 질환입니다. 집먼지, 반려동물, 곰팡이 및 환절기 알레르기와 같은 알레르기비염의 외부 요인에 대해 알아보십시오.
1. Van Cauwenberge et al. Comparison of the efficacy, safety and quality of life provided by fexofenadine hydrochloride 120 mg. Clin Exp Allergy. 2000.30(6);891-899
2. Mark E et al. Efficacy and tolerability of 2nd and 3rd gen antihistamines. ANNALS OF ALLERGY, ASTHMA & IMMUNOL. 2010.104;518–522
3. Fein MN et al. CSACI position statement_Newer generation H1-antihistamines (3rd generation) are safer than first-generation H1-antihistamines. Allergy Asthma Clin Immunol. 2019.15(61);1-6
4. Anne K et al. Second-and third-generation antihistamines. DERMATOLOGIC THERAPY. 2000.13;327–336
5. Thomas B et al. Next generation antihistamines therapeutic rationale accomplishments and advances. Expert Opinion on Investigational Drugs. 2002.11(6);807-817
6. Day J H et al. Onset of action, efficacy, and safety of a single dose of fexofenadine hydrochloride. Ann Allergy Asthma Immunol. 1997.79;533-540
7. Smith SM et al. Fexofenadine: biochemical, pharmacokinetic and pharmacodynamic properties and its unique role in allergic disorders. Expert Opinion on Drug Metabolism & Toxicology. 2009.5(7);813-822
8. Peter H et al. Double-blind, placebo-controlled study comparing the efficacy and safety of fexofenadine and cetirizine in seasonal AR. J ALLERGY CLIN IMMUNOL. 1999.104(5);927-933
9. 2023 소비자가 뽑은 올해의 브랜드대상, 2년 연속 수상 (알레르기비염 치료제 부문) 사단법인 한국방송신문연합회 주관
10. Brożek JL et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J ALLERGY CLIN IMMUNOL. 2017.140(4);950-958
[Study Design1] This was a multinational, multicentre, double-blind, parallel group, randomized, placebo-controlled study. Following a placebo run-in phase of 3±7 days (the baseline period), patients with SAR were randomized to receive one capsule of either fexofenadine HCl 120mg(n=232), loratadine 10mg(n=228), or placebo(n=225), once each morning for 14 days. The primary efficacy parameter was the change in the mean 24-h reflective TSS during the double-blind treatment period from that during the baseline period.
[Study Design6] This study was of a randomized, placebo-controlled, double-blind, parallel design. The purpose of this study was to characterize the time to onset of clinically important relief of symptoms of allergic rhinitis in subjects taking single doses of either 60 mg or 120 mg fexofenadine HCl, or placebo, after exposure to ragweed pollen in a controlled environment. Other objectives were to assess the efficacy and safety of single doses of fexofenadine HCl.
[Study Design8] A multicenter, double-blind, parallel-group, placebo-controlled trial compared the efficacy and safety of fexofenadine HCl (120 and 180 mg administered once daily) and cetirizine (10 mg once daily) in the treatment of seasonal allergic rhinitis. The primary efficacy variable was the change in mean 24-hour reflective TSS during the treatment period in relation to the single blind placebo lead-in phase.
※3세대 성분들의 경우, 2세대의 개선 성분으로 2세대에 포함시키는 경우도 있습니다.
.webp/jcr:content/Secondary%20image%20(1).webp 400w,/.imaging/webp/sanofi-chc/img-w500/dam/allegra-kr/pages/home/Secondary-image-(1).webp/jcr:content/Secondary%20image%20(1).webp 500w,/.imaging/webp/sanofi-chc/img-w600/dam/allegra-kr/pages/home/Secondary-image-(1).webp/jcr:content/Secondary%20image%20(1).webp 600w,/.imaging/webp/sanofi-chc/img-w700/dam/allegra-kr/pages/home/Secondary-image-(1).webp/jcr:content/Secondary%20image%20(1).webp 700w,/.imaging/webp/sanofi-chc/img-w800/dam/allegra-kr/pages/home/Secondary-image-(1).webp/jcr:content/Secondary%20image%20(1).webp 800w,/.imaging/webp/sanofi-chc/img-w900/dam/allegra-kr/pages/home/Secondary-image-(1).webp/jcr:content/Secondary%20image%20(1).webp 900w)
.webp/jcr:content/Image%203%20(1).webp 400w,/.imaging/webp/sanofi-chc/img-w500/dam/allegra-kr/pages/home/Image-3-(1).webp/jcr:content/Image%203%20(1).webp 500w,/.imaging/webp/sanofi-chc/img-w600/dam/allegra-kr/pages/home/Image-3-(1).webp/jcr:content/Image%203%20(1).webp 600w,/.imaging/webp/sanofi-chc/img-w700/dam/allegra-kr/pages/home/Image-3-(1).webp/jcr:content/Image%203%20(1).webp 700w,/.imaging/webp/sanofi-chc/img-w800/dam/allegra-kr/pages/home/Image-3-(1).webp/jcr:content/Image%203%20(1).webp 800w,/.imaging/webp/sanofi-chc/img-w900/dam/allegra-kr/pages/home/Image-3-(1).webp/jcr:content/Image%203%20(1).webp 900w)
.webp/jcr:content/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon%20(1).webp 400w,/.imaging/webp/sanofi-chc/img-w500/dam/allegra-kr/pages/home/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon-(1).webp/jcr:content/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon%20(1).webp 500w,/.imaging/webp/sanofi-chc/img-w600/dam/allegra-kr/pages/home/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon-(1).webp/jcr:content/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon%20(1).webp 600w,/.imaging/webp/sanofi-chc/img-w700/dam/allegra-kr/pages/home/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon-(1).webp/jcr:content/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon%20(1).webp 700w,/.imaging/webp/sanofi-chc/img-w800/dam/allegra-kr/pages/home/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon-(1).webp/jcr:content/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon%20(1).webp 800w,/.imaging/webp/sanofi-chc/img-w900/dam/allegra-kr/pages/home/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon-(1).webp/jcr:content/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon%20(1).webp 900w,/.imaging/webp/sanofi-chc/img-w1200/dam/allegra-kr/pages/home/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon-(1).webp/jcr:content/Allegra_Allergy_Stock_Image_Kid_indoor_sofa_cat_DigitalMedia_DigitalMedia_Gold_Global_202501_3_11zon%20(1).webp 1200w)
.webp/jcr:content/Allegra_Wonka_Article_Images_Cold%20Or%20Allergie%20_02_Global%20(2).webp 400w,/.imaging/webp/sanofi-chc/img-w500/dam/allegra-kr/pages/home/Allegra_Wonka_Article_Images_Cold-Or-Allergie-_02_Global-(2).webp/jcr:content/Allegra_Wonka_Article_Images_Cold%20Or%20Allergie%20_02_Global%20(2).webp 500w,/.imaging/webp/sanofi-chc/img-w600/dam/allegra-kr/pages/home/Allegra_Wonka_Article_Images_Cold-Or-Allergie-_02_Global-(2).webp/jcr:content/Allegra_Wonka_Article_Images_Cold%20Or%20Allergie%20_02_Global%20(2).webp 600w,/.imaging/webp/sanofi-chc/img-w700/dam/allegra-kr/pages/home/Allegra_Wonka_Article_Images_Cold-Or-Allergie-_02_Global-(2).webp/jcr:content/Allegra_Wonka_Article_Images_Cold%20Or%20Allergie%20_02_Global%20(2).webp 700w,/.imaging/webp/sanofi-chc/img-w800/dam/allegra-kr/pages/home/Allegra_Wonka_Article_Images_Cold-Or-Allergie-_02_Global-(2).webp/jcr:content/Allegra_Wonka_Article_Images_Cold%20Or%20Allergie%20_02_Global%20(2).webp 800w,/.imaging/webp/sanofi-chc/img-w900/dam/allegra-kr/pages/home/Allegra_Wonka_Article_Images_Cold-Or-Allergie-_02_Global-(2).webp/jcr:content/Allegra_Wonka_Article_Images_Cold%20Or%20Allergie%20_02_Global%20(2).webp 900w)