알레그라®(펙소페나딘)는 효과 빠른 3세대 항히스타민제입니다.1-4,5
우리 몸은 외부에서 침입한 물질(알레르겐)에 대해 방어 작용을 합니다. 그런데 이 과정에서 히스타민이라는 물질이 과도하게 분비되면 콧물, 재채기, 코막힘, 가려움 등의 알레르기 비염 반응이 나타나게 됩니다. 항히스타민제는 바로 이 히스타민의 작용을 차단하여 알레르기 비염 증상을 완화시켜주는 약물입니다.
항히스타민제는 길항작용*으로 H1 수용체 부위에 히스타민과 경쟁적으로 작용함으로써 알레르기비염 증상을 완화시킵니다. 알레그라®는 바로 이 3세대 항히스타민제에 속하며, 펙소페나딘 성분이 히스타민 작용을 효과적으로 차단하여 알레르기 비염 증상을 빠르게 완화시켜 줍니다. 대부분의 알레르기 비염 환자는 약물 치료를 통해 증상 및 삶의 질을 개선할 수 있습니다.※3세대 성분들의 경우, 2세대의 개선 성분으로 2세대에 포함시키는 경우도 있습니다.
알레그라®는 바로 이 3세대 항히스타민제에 속하며, 펙소페나딘 성분이 히스타민 작용을 효과적으로 차단하여 알레르기 비염 증상을 빠르게 완화시켜 줍니다. 대부분의 알레르기 비염 환자는 약물 치료를 통해 증상 및 삶의 질을 개선할 수 있습니다.
※3세대 성분들의 경우, 2세대의 개선 성분으로 2세대에 포함시키는 경우도 있습니다.
H1 항히스타민제 작용 기전11
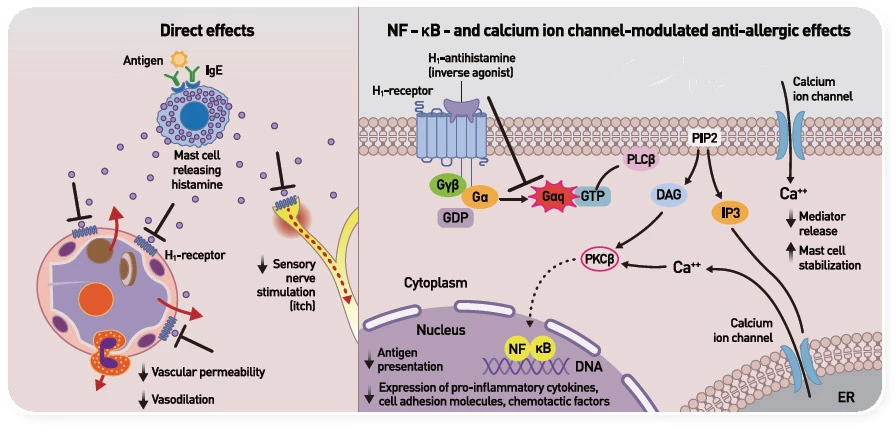
Adapted from Simons FE, et al. Histamine and H1-antihistamines Celebrating a century of progress. 2011
* 수용체와 결합하여 작용을 억제함
1세대, 2세대, 3세대... 항히스타민제, 뭐가 다른 걸까요?
항히스타민제는 개발 순서, H1 affinity, 안전성 프로파일 등에 따라 1 세대, 2 세대, 그리고 3 세대로 구분되며 세대가 진화함에 따라 점차적으로H1 히스타민 수용체에 대한 선택성이 높아지고 항-알레르기 효과가 높아졌습니다.
알레그라® 정 120mg 은 약국에 정식 출시한 첫 3세대 항히스타민제 일반의약품입니다.
1세대 항히스타민제 종류는 개발 초기 단계의 약물로, 졸음이나 입 마름 등의 부작용이 나타날 수 있으며, 약효 지속 시간이 짧아 하루에 여러 번 복용해야 하는 번거로움이 있었습니다.
2세대 항히스타민제는 1세대에 비해 부작용이 줄고, 약효 지속 시간도 길어져 하루 1~2회 복용으로 충분한 경우가 많습니다. 다만, 일부에서는 여전히 졸음이나 피로감 같은 증상이 나타날 수 있습니다.
3세대 항히스타민제는 최신 기술로 개발된 약물로, 1세대, 2세대에 비해 졸음 등의 부작용이 현저히 감소했습니다. 또한 약효가 빠르게 나타나고 오래 지속되며, 다른 약물과의 상호작용도 적다는 장점이 있습니다.
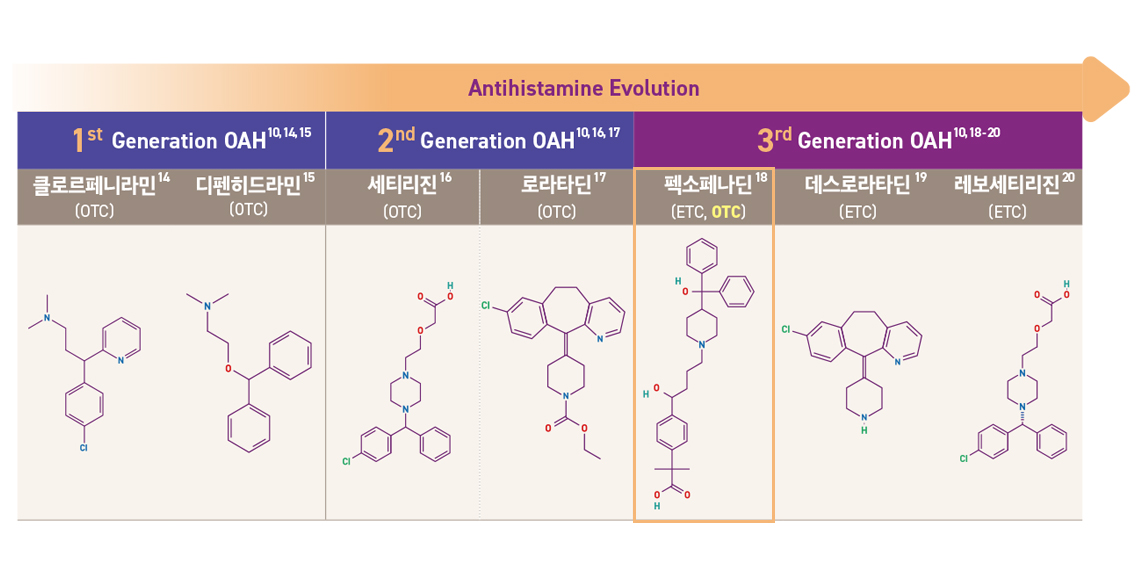
졸음 걱정을 줄인 3세대 항히스타민제, 펙소페나딘. 3세대 항히스타민제 성분의 펙소페나딘은 꽃가루, 반려동물 털, 먼지, 환절기 알레르기와 관련된 알레르기비염 증상 완화에 효과적입니다. 알레그라®정 120mg의 경우, 다양한 알레르기비염 증상 완화(코막힘, 맑은 콧물, 재채기, 눈, 코 가려움증 등)에 적응증을 가지고 있습니다.
알레그라®의 펙소페나딘(Fexofenadine)은 혈액-뇌 장벽(blood-brain barrier, BBB)을 통과하지 않으므로, 항히스타민제 성분군에 해당합니다.
진정 작용에 따른 항히스타민제 분류24
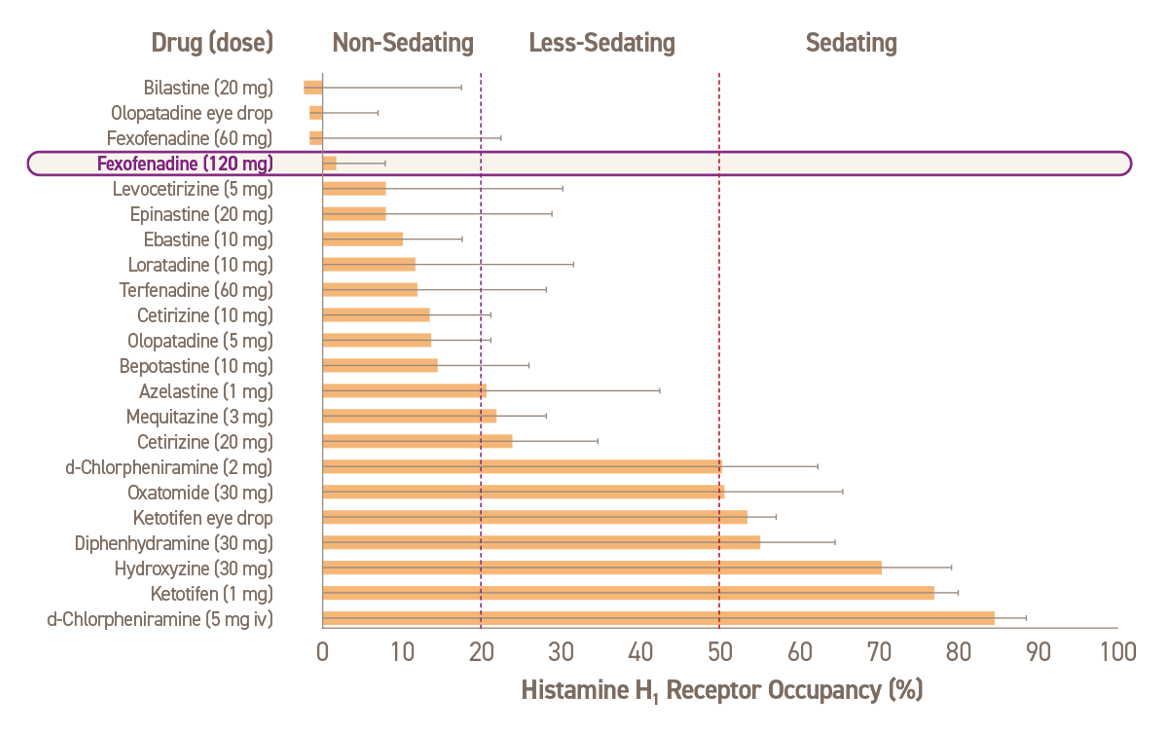
Adapted from Kawauchi H, et al. Antihistamines for AR Treatment from the Viewpoint of Nonsedative Properties. 2019
1. Mark E et al. Efficacy and tolerability of 2nd and 3rd gen antihistamines. ANNALS OF ALLERGY, ASTHMA & IMMUNOL. 2010.104;518–522
2. Fein MN et al. CSACI position statement_Newer generation H1-antihistamines (3rd generation) are safer than first-generation H1-antihistamines. Allergy Asthma Clin Immunol. 2019.15(61);1-6
3. Anne K et al. Second-and third-generation antihistamines. DERMATOLOGIC THERAPY. 2000.13;327–336
4. Thomas B et al. Next generation antihistamines therapeutic rationale accomplishments and advances. Expert Opinion on Investigational Drugs. 2002.11(6);807-817
5. Day J H et al. Onset of action, efficacy, and safety of a single dose of fexofenadine hydrochloride. Ann Allergy Asthma Immunol. 1997.79;533-540
6. Smith SM et al. Fexofenadine: biochemical, pharmacokinetic and pharmacodynamic properties and its unique role in allergic disorders. Expert Opinion on Drug Metabolism & Toxicology. 2009.5(7);813-822
7. Peter H et al. Double-blind, placebo-controlled study. Comparing the efficacy and safety of fexofenadine and cetirizine in seasonal AR. J ALLERGY CLIN IMMUNOL. 1999.104(5);927-933
8. Meltzer EO et al. Once daily fexofenadine HCl improves quality of life and reduces work and activity impairment in patients with seasonal allergic rhinitis. Mayo Clin Proc. 1999.84(4);311-317
9. Bousquet J et al. Allergic Rhinitis. Nat Rev Dis Primers. 2020.6(95);1-17
10. Olasińska-Wiśniewska A et al. Cardiovascular safety of antihistamines. Post ę py Dermatologii i Alergologii. 2014.31(3);182-186
11. Simons FE et al. Histamine and H1-antihistamines Celebrating a century of progress. J ALLERGY CLIN IMMUNOL. 2011.128(6); 1139-1150
12. 약학정보원. 약물백과 항히스타민제 (Accessed May 13 2023)
13. Data on file. Allegra tablet 120mg Sales data from IQVIA. Q2 2018 - Q1 2023 (ATC4 code R06A0)
14. 약학정보원. 약물백과 클로르페니라민 (Accessed May 19 2023)
15. 약학정보원. 약물백과 디펜히드라민 (Accessed May 19 2023)
16. 약학정보원. 성분정보 세티리진염산염 (Accessed May 19 2023)
17. 약학정보원. 약물백과 로라타딘 (Accessed May 19 2023)
18. 약학정보원. 성분정보 펙소페나딘염산염 (Accessed May 19 2023)
19. 약학정보원. 성분정보 데스로라타딘 (Accessed May 19 2023)
20. 약학정보원. 약물백과 레보세티리진 (Accessed May 19 2023)
21. Brożek JL et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J ALLERGY CLIN IMMUNOL. 2017.140(4), 950-958
22. Van Cauwenberge et al. Comparison of the efficacy, safety and quality of life provided by fexofenadine hydrochloride 120 mg. Clin Exp Allergy. 2000. 30(6);891-899
23. 식품의약품안전처. 의약품통합정보시스템. 알레그라정120(일반의약품) (Accessed Jun 19th, 2023)
24. Kawauchi H et al. Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties. Int. J. Mol. Sci. 2019;20(213) 1-17
25. Data on file. Allegra tablet 120mg Sales data from IQVIA. 2022.03 - 2023.01 (ATC4 code R06A0)
[Study Design5] This study was of a randomized, placebo-controlled, double-blind, parallel design. The purpose of this study was to characterize the time to onset of clinically important relief of symptoms of allergic rhinitis in subjects taking single doses of either 60 mg or 120 mg fexofenadine HCl, or placebo, after exposure to ragweed pollen in a controlled environment. Other objectives were to assess the efficacy and safety of single doses of fexofenadine HCl.
[Study Design7] A multicenter, double-blind, parallel-group, placebo-controlled trial compared the efficacy and safety of fexofenadine HCl (120 and 180 mg administered once daily) and cetirizine (10 mg once daily) in the treatment of seasonal allergic rhinitis. The primary efficacy variable was the change in mean 24-hour reflective TSS during the treatment period in relation to the single blind placebo lead-in phase.
[Study Design22] This was a multinational, multicentre, double-blind, parallel group, randomized, placebo-controlled study. Following a placebo run-in phase of 3±7 days (the baseline period), patients with SAR were randomized to receive one capsule of either fexofenadine HCl 120mg(n=232), loratadine 10mg(n=228), or placebo(n=225), once each morning for 14 days. The primary efficacy parameter was the change in the mean 24-h reflective TSS during the double-blind treatment period from that during the baseline period.
※3세대 성분들의 경우, 2세대의 개선 성분으로 2세대에 포함시키는 경우도 있습니다.
MAT-KR-2301159-v1.0-07/2023

